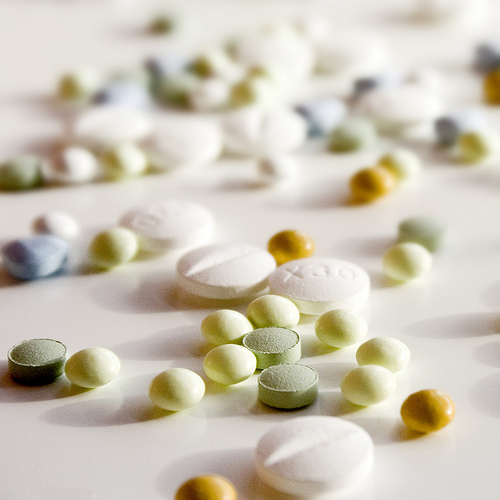
 Scientists know a lot about infectious diseases. But a new study in the Archives of Internal Medicine finds that the treatment guidelines created by the Infectious Diseases Society of America (IDSA) are based on imperfect evidence. Only one in seven treatment recommendations relied on evidence from a randomized controlled trial, the gold standard in medicine.
Scientists know a lot about infectious diseases. But a new study in the Archives of Internal Medicine finds that the treatment guidelines created by the Infectious Diseases Society of America (IDSA) are based on imperfect evidence. Only one in seven treatment recommendations relied on evidence from a randomized controlled trial, the gold standard in medicine.
In a randomized trial, participants are randomly assigned to one of two groups—the “treatment” group or the control group. The control group receives the standard of care, say ibuprofen for a headache. The treatment group receives whatever new-fangled drug or intervention the researchers want to test, say meditation or Valium.
At first blush, this apparent lack of high-quality evidence may seem like cause for outrage. But ask any medical researcher, and they will tell you that clinical trials are crazy expensive. In some cases, they aren’t even possible. And in other cases, they’re just plain stupid: We don’t need a clinical trial, the authors point out, to tell us that staying far away from ticks cuts our risk of contracting Lyme disease, nor do we need a clinical trial to tell us that nurses and doctors should wash their hands to prevent the spread of infections. Continue reading

 First week of January. Like everybody else in America, I’m on a diet.
First week of January. Like everybody else in America, I’m on a diet.
