Jessa Gamble is embedded in an experimental evolution lab at the University of Ottawa.
Hope Jahren writes that you can hear corn growing in the Midwest. It sounds like the collective rustle of husks adjusting to accommodate the day’s inch of growth. I am tempted to put a microphone in the incubator. Would my bacteria whine or hiss as they grow? Amplified, would there be a little crackle or snap as daughter cells break free of each other?
The incubator is the one place we want things to grow. Everywhere else those same organisms become weeds. “Contamination” is another word for growing in the wrong place, and fighting it is the whole game in this lab. Our implements of war are open flame, alcohol, and paranoia—the stuff of revolution.
Things I learned this week:
-
One does not simply “go for lunch” and expect your numbers to be as you left them. Your bacteria ate lunch, too.
-
Expect to spend significant time as a human blender-slash-cocktail mixer.
-
The tip ejector release mechanism on the pipette is easily mistaken by a blindly probing thumb for the plunger. This can lead you to dump a great honking piece of plastic into a tube that was meant to receive 6 micro-litres of fluid. Avoid this.
This weeks’ focus in my lab work was not useful science, per se, but the hope is that it’s useful for science. The task was a calibration project, the results of which are to be posted for everyone’s future reference. When bacteria grow for any length of time, it isn’t practical to count cells individually. For one thing, the numbers are overwhelming, and for another, they tend to clump together in mats that make it impossible to discern their numbers by eye.
One way to estimate the growth rate is to first take an optical density read, which shines light through your bacteria in their medium. Once you account for the fogginess of the nutrient-rich medium itself, the rest of the haze is due to cells floating around. To figure out how many bacteria you’ve grown, you get that optical density (OD) reading and then, based on previous experience, you decide how far you should to dilute your sample before you spread it on an agar plate, distributing cells—or “colony-forming units” that may be small clusters of cells—over the surface of the agar. In a few days those tiny pioneers will have cloned themselves into little settlements visible to the eye, and you can count them.
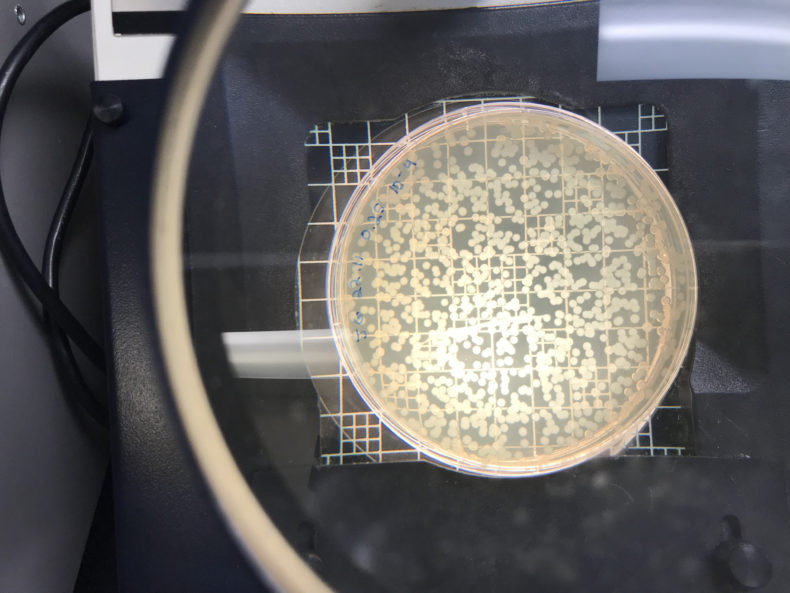
If you didn’t dilute enough, you get a bunch of overlapping blobs that are hard to count:
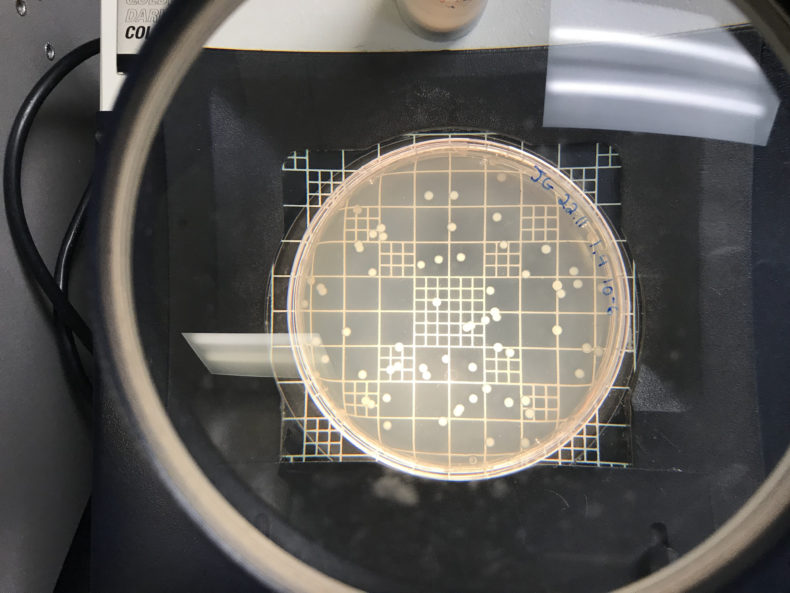
If you diluted too much, you don’t have enough of a count to really make an accurate extrapolation:
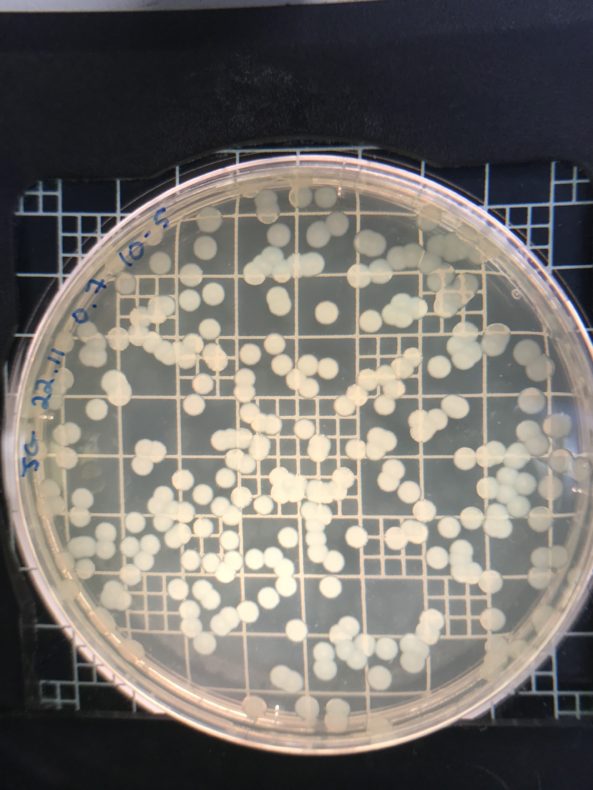
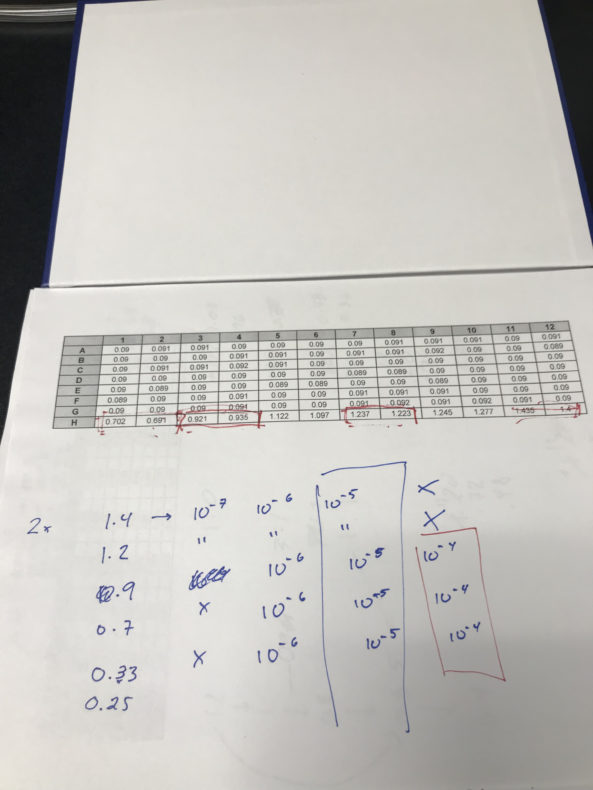
But if you get it just right, you’ve got somewhere between 100 and 300 colonies.The trick is to get a meaningful number of colonies forming on your plates without having so many that you can’t tell them apart. That’s all in your dilution judgment call. Felipe and I set out to take the guesswork out of that dilution decision. We would make a chart that describes how many colonies you get when you take a given optical density and dilute the sample by a given order of magnitude.
Once you have a countable number of colonies, you can calculate to correct for the dilution and reconstruct the original number in your sample. If you’ve diluted the medium by 106, for example, you can assume the original equivalent volume held about a million times as many. My 36 plates have been seeded and spread with diluted cultures, and the colonies on them have grown into nicely visible blobs. Tomorrow’s task is to count them. If the results make sense, I will proudly post them on the lab wall. If not, it’s time to try again.
Current Reading (open to suggestions)
Big Questions in Ecology and Evolution, by Thomas N. Sherratt & David M. Wilkinson (thanks Partha!)
Laboratory Life: The Construction of Scientific Facts, by Bruno Latour and Steve Woolgar
The Tangled Tree: A Radical New History of Life, by David Quammen
The Data Scientist’s Toolbox, through Johns Hopkins on Coursera