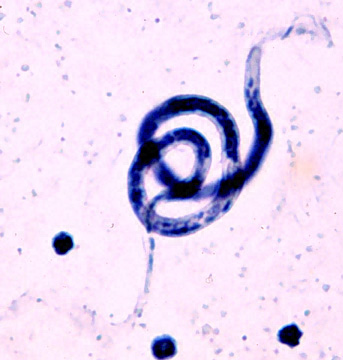
 This worm is born to travel. It begins life in human lymph, only to seep out of the lymphatic vessels into the grimy fluid that bathes our organs. From there, it drifts into the blood stream. During the day, it keeps to deep veins. Once darkness falls, it migrates up to the skinny veins just under the skin.
This worm is born to travel. It begins life in human lymph, only to seep out of the lymphatic vessels into the grimy fluid that bathes our organs. From there, it drifts into the blood stream. During the day, it keeps to deep veins. Once darkness falls, it migrates up to the skinny veins just under the skin.
Then one lucky night, a mosquito will find the sleeping human and feast on its blood. The worm will end up in the insect’s gut and, eventually, in its muscles. It will reach adolescence there, and then travel to the mosquito’s head, stinger and, finally, to the next person the insect bites. From the blood stream, the worm will find its way back to the lymph to mate and, after such a long journey, retire. It will stay there for six to eight years, the rest of its life, and pump out millions of new little worms to embark on the same cross-species adventure.
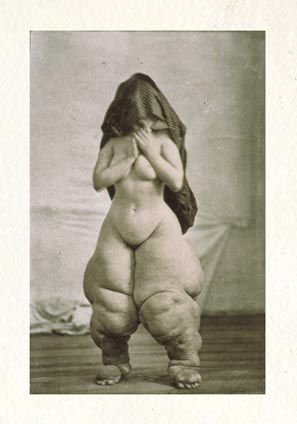
Unfortunately, the health of these worms, called parasitic filarial nematodes, is in direct conflict with that of their human hosts. The worms slowly accumulate inside of people, eventually clogging lymph nodes and causing the extreme swelling, discoloration and deformity known as elephantiasis. More than 120 million people in 72 countries are infected with the disease, formally called lymphatic filariasis, leaving some 40 million incapacitated.
There is no cure, but there is a potent treatment called ivermectin. The drug paralyzes juvenile worms swimming in blood, making them much more vulnerable to attack by the human immune system. But ivermectin has little effect on the long-living adult worms in the lymph. To target those, doctors rely on two other drugs, diethylcarbamazine and albendazole.
In the past decade or so, several large pharmaceutical companies have worked with the World Health Organization and the Gates Foundation to widely distribute these drugs for free in developing countries. “In certain areas of the world, these compounds have been incredibly effective at reducing transmission,” says Tilde Carlow, a parasitologist at New England Biolabs.
But the drugs are not ideal. First problem: In some parts of Africa, many people are also infected with a different parasite, called the loa loa worm, that can cause adverse reactions to diethylcarbamazine and ivermectin.
Then there’s the more controversial issue of ivermectin resistance, which would play out like this: Some worms carry genetic glitches that just happen to help them evade the drug. Over time their descendants will thrive, eventually making ivermectin useless. Widespread resistance has already happened in worms living inside of farm animals, which have been exposed to the drug much longer than humans have. “So there’s a lot of caution and concern that when you’re using this drug in these massive drug campaigns for humans something similar is going to happen,” Carlow says.
A study published last year gave the first evidence that ivermectin resistance is indeed happening in people. The researchers focused on Onchocerca volvulus, a worm that causes another nasty tropical disease called onchocerciasis, or “river blindness.” Some 25 percent of infected people in Ghana — where ivermectin is used heavily — carry worms that respond poorly to the drug, the study found. Researchers are still hotly debating the extent of the resistance, and the speed of its potential spread, Carlow says. “But the implications are huge.”
Which is all to explain why, for the past 28 years, Carlow and her colleagues have been trying to develop new ways to eliminate these parasites. Because of their complicated life cycles, the worms are extremely tricky to grow in the lab (it involves gerbils and mosquitos). But the genomic revolution of the past few years has uncovered a lot of information about the worms’ genes and those of a closely related species, Caenorhabditis elegans, which is easy to keep alive and ubiquitous in genetic research.
Why is it helpful to decode their genomes? “It helps us explore what these worms are made of, and find their Achilles’ heel,” Carlow says. For example, by looking at the genetic code, scientists discovered that one parasite carries around a bacteria called Wolbachia that somehow helps the worms survive. That opened up a whole new line of attack against the worms: antibacterial drugs. More recently, Carlow’s lab has identified two compounds that target a crucial enzyme inside of Wolbachia. When that’s knocked down, so is the bacteria, and so is the wayfaring worm.
One way to deliver diethylcarbamazine in areas where the disease is endemic is to lace the salt supply. That’s what they’re doing in Guyana (though it probably wouldn’t be a good idea in Africa for the reason you mention). I wrote about it here: http://www.geotimes.org/june08/article.html?id=feature_salt.html
I remember that story, Cassie! Cool beans.
Another issue is that in many of these countries, the coverage of distribution is paltry, I can say this about india, that while i have witnessed and partaken in 2 rounds of mass distribution, the coverage is far less than is needed for effective prevention.
And with the rise of clean water/sanitation facilities, I wonder if the answer lies in mass medication when the same funds expended in prevention might pay bigger returns
The first paragraph of this piece read like a poem. Loved the way you describe the worm’s path!
Aw, thanks, Meg.